Real people, real stories
Hear from others about their experiences using MIEBO.
Although you may not have heard about tear evaporation, it’s the #1 cause of those pesky dry eye symptoms. Until MIEBO, there hasn’t been a prescription eye drop that specifically targets it.

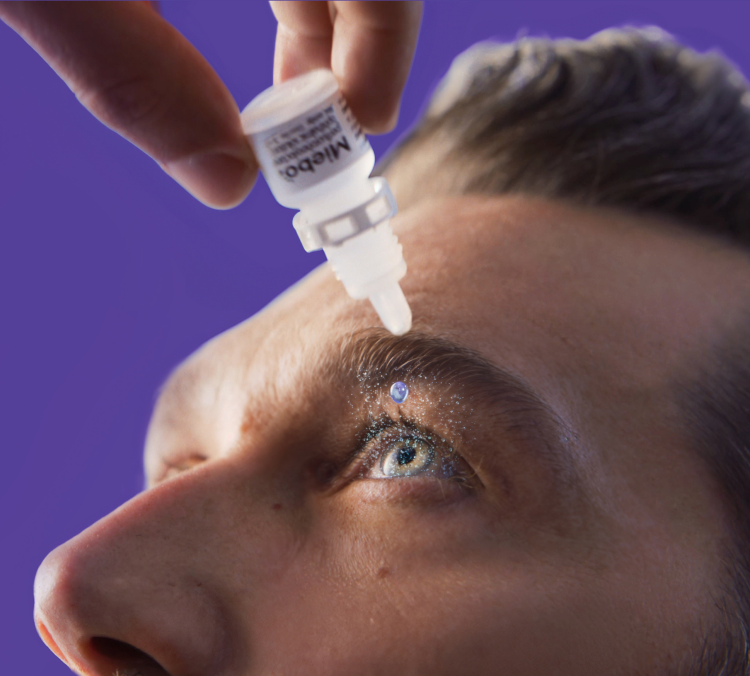
MIEBO is a different type of eye drop for dry eye. It’s the first and only prescription eye drop that directly targets too much tear evaporation.
When you place a drop of MIEBO in your eye, it spreads evenly and quickly to form a protective layer.
This reduces evaporation, so you can keep more of your own tears.
The exact way MIEBO works is unknown.






1 of 5 chapters



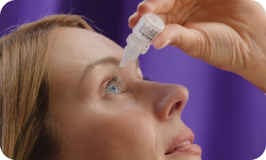
MIEBO® (perfluorohexyloctane ophthalmic solution) is used to treat the signs and symptoms of dry eye disease.
IMPORTANT SAFETY INFORMATION
Remove contact lenses before using MIEBO and wait for at least 30 minutes before reinserting.
Please stay tuned for Important Safety Information about MIEBO at the end of this video.
Let’s take a closer look at what leads to too much tear evaporation.
The why behind tear evaporation
On the outside of your eye, there’s something called the tear film. The tear film is made up of mucus, water, and an oil, or lipid, called meibum.
In a healthy eye, the meibum prevents the water from evaporating too quickly. This helps protect the surface of the eye and keeps it from drying out. In a dry eye, the meibum isn't effective, and too many tears evaporate.
Why there isn’t enough meibum
Little glands called meibomian glands make the meibum. Every time you blink, the meibum is spread across the surface of your eye.
When the meibomian glands don’t work as they should, they can’t create enough meibum. This is called meibomian gland dysfunction, or MGD. MGD, which leads to too much tear evaporation, is a cause of dry eye for about 9 out of 10 people.
Looking at screens can make you blink less often and less fully—and makes your meibum less effective.
A cycle of damage
Without enough effective meibum, tears evaporate too quickly.
When there aren’t enough tears, the cells on the surface of your eye dry up and flake off faster than they can be replaced.
This, in turn, creates a roughness on the eye’s surface. Not having enough tears can also cause friction when you blink, creating more damage... and more symptoms.
Although your eyes may try to produce more tears to make up for the ones that have evaporated, the new tears still evaporate too quickly without enough meibum to keep them in, which continues the overproduction of tears. That continual tear production is why your eyes may feel wet or teary when you have dry eye.
The MIEBO difference
MIEBO is the first and only prescription eye drop that helps protect against too much tear evaporation.
When you place a drop of MIEBO in your eye, it spreads evenly and quickly to form a protective, long-lasting layer that reduces tear evaporation.
MIEBO is a small, comfortable drop that mimics the way your own meibum helps prevent tear evaporation.
Keeping more of your own tears may reduce dry eye symptoms and can help heal the surface of the eye when you keep using it as directed.
If your dry eye symptoms keep coming back, it could be too much tear evaporation. Talk to your eye doctor about prescription MIEBO.
INDICATION
MIEBO is used to treat the signs and symptoms of dry eye disease.
IMPORTANT SAFETY INFORMATION
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information for MIEBO at MIEBO.com.
1 of 5 chapters





MIEBO stories
Hear from others about their experiences using MIEBO.
MIEBO® (perfluorohexyloctane ophthalmic solution) is used to treat the signs and symptoms of dry eye disease.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Click here for full Prescribing Information for MIEBO.
MIEBO® (perfluorohexyloctane ophthalmic solution) is used to treat the signs and symptoms of dry eye disease.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Click here for full Prescribing Information for MIEBO.